Mcpba H3O Reaction. We identified it from obedient source. An acid / base reaction.
And we also have this quaternary ammonium ion, which is going to go ahead and get deeper donated by this hydroxide. The protocol offers use of readily available starting materials and broad substrate scope. Attack of the nucleophilic water molecule on the electrophilic carbocation creates an oxonium ion.
Memorize Reaction, Orientation Where Appropriate, Stereochemistry Where Appropriate, And Mechanism Where Appropriate.
Orientation stereo mechanism 1 hrb r (no peroxides) c The transformation is of value because, as we know, oxacyclopropanes are versatile. The pi electrons act as a lewis base.
Mcpbacl2, H2Obr2, H2Oh3O+, Hoch31.Bh3.Thf 2.H2O2, Naohh2, Pt1.Hg (Oac)2, H2O 2.Nabh4Hbrhihcloso4, H2O2H3O+1.Mcpba 2.H3O+Hbr, Roor, Heatbr2Warm, Concentrated Kmno41.O3 2.
It provides the arrow pushing drawing that shows how an. Which is the correct sequence of reaction steps necessary to complete the following transformation? 7:39 m l 65% on.masteringchemistry.com :d
We Identified It From Obedient Source.
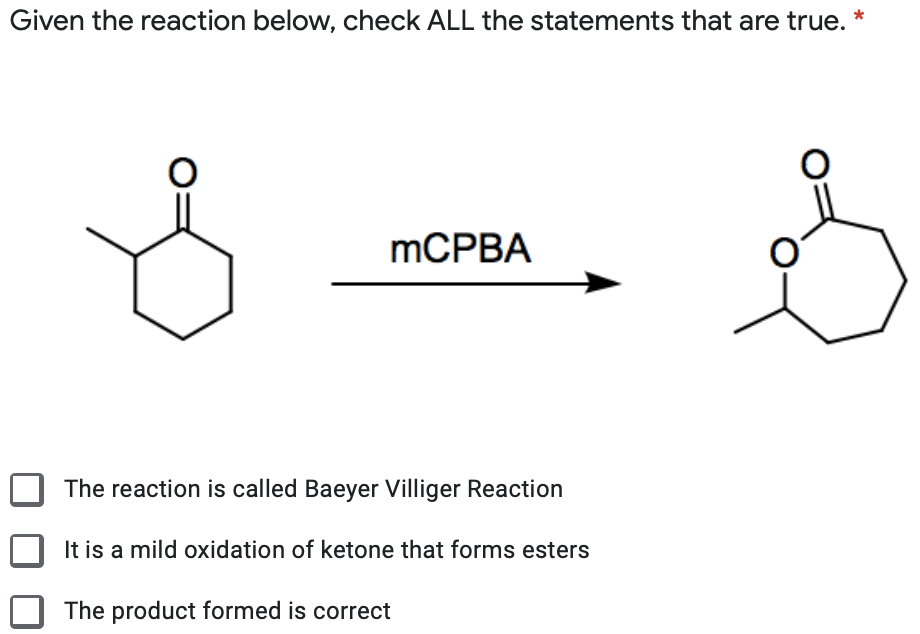
This problem is asking us to predict the major product of this reaction. That's hydrogen associate with the beta carbon. This is a rare example of a reaction that results in the oxidation of a ketone.
Mechanism For Reaction Of Alkenes With H 3 O + Step 1:
So we see that we have heat involved. The solution aged three o plus and, oh, age minus, they could put they conform water. The other product of the reaction is a carboxylic acid.
And We Also Have This Quaternary Ammonium Ion, Which Is Going To Go Ahead And Get Deeper Donated By This Hydroxide.
When the following alkene is reacted with mcpba, an unsymmetrical epoxide is formed. We say yes this kind of reaction with h3o graphic could possibly be the most trending subject following we allowance it in google help or facebook. Strong and weak nucleophiles open the epoxide ring from the back side.